The Bio-Engineered Human
The scene is an apex of science fiction drama: a dying man, Roy Batty, stands before his creator, Dr. Eldon Tyrell, in a desolate, futuristic Los Angeles. The year is 2019, according to the film’s own internal chronology, and the question is as old as philosophy itself: the desire for "more life." Batty, a Nexus-6 Replicant, confronts Tyrell about the built-in, non-negotiable four-year expiration date on his existence, a timeline dictated not by failing mechanical parts, but by a catastrophic biological certainty that makes his body fail prematurely.1 Tyrell’s response—that Batty’s existence, like a blazing light, was necessarily short-lived due to the intensity of its engineering—serves as the tragic hinge upon which the entire narrative swings.
It is paramount to understand the nature of the Replicant as presented in Blade Runner. They are not, contrary to many popular interpretations and science fiction tropes, complex machines or Terminator-style androids with positronic brains. They are, instead, the successful culmination of bio-engineering: complex, sentient, genetically-engineered human-like organisms, grown from the fetus to maturity through artificial wombs.2 Their bodies are composed of flesh, blood, organs, and DNA, making the issue of their mortality a profound question of genetics and medicine, not robotics. The four-year limit is therefore a function of accelerated, catastrophic cellular senescence—a genetic time bomb wired into their very chromosomes—and not a simple mechanical breakdown.
Tyrell’s ultimate refusal to extend Batty’s life is founded on a scientific dogma: the alteration required to fix the flaw is a "fatal alteration." While this absolute claim was a plausible scientific barrier when the film was conceived, the intervening decades have seen breakthroughs that challenge this very premise. The advent of precision genetic tools, chiefly the CRISPR-Cas9 system, has fundamentally changed what we understand to be possible in biological engineering.3 Where Tyrell saw only insurmountable risk, contemporary genomic science offers tools for high-precision systemic editing. The scientific claim of "fatal alteration" is now largely obsolete; however, Tyrell’s underlying warning regarding the unfathomable complexity of life—and the corporate mandate for control that superseded compassion—remains tragically relevant.4
Notes
1 Scott, Ridley (Director). Blade Runner (1982). Warner Bros. Film depicting the confrontation between Roy Batty and Eldon Tyrell regarding the accelerated lifespan.
2 K. W. Jeter. Blade Runner 2: The Edge of Human (1995). Bantam Books. Expanded narrative defining the Nexus-6 and Nexus-7 series as purely biological entities.
3 Doudna, J. A., & Charpentier, E. (2020). Genome engineering using CRISPR-Cas9 system. Nobel Lecture. Detailing the foundational science of clustered regularly interspaced short palindromic repeats and Cas9 protein.
4 Fukuyama, F. (2002). Our Posthuman Future: Consequences of the Biotechnology Revolution. Farrar, Straus and Giroux. Discussion on the ethical and societal control mechanisms inherent in advanced biological engineering.The Bio-Engineered Human
The Blade Runner Conundrum: Tyrell’s Dogma
The tension within the Tyrell corporate headquarters reaches its peak when Roy Batty delivers his desperate, concise demand: "I want more life, father." Eldon Tyrell’s response, delivered with the calculated detachment of a creator defending an engineering principle, is the narrative's most famous scientific alibi: "The light that burns twice as bright burns half as long—and you have burned so very, very brightly, Roy." Batty’s existence, Tyrell suggests, is an optimized system, one that trades longevity for performance, power, and enhanced sensory input, encapsulated by the replicant’s superior strength and reflexes.5 This dialogue moves the tragic flaw out of the realm of mere sabotage and places it firmly within the realm of a forced, inevitable biological mechanism—the "fatal alteration" that Tyrell insists cannot be undone.
Scientifically, Batty’s accelerated decay is a textbook example of severe, systemic accelerated cellular senescence.6 This is the process where cells cease to divide and enter an irreversible state of dormancy, contributing significantly to the pathology of aging in all biological life. For the Nexus-6, this process is dramatically compressed from a human lifetime of roughly 80 years down to 1,460 days. The universally accepted biological clock dictating cellular division and aging is the telomere, the protective cap found on the end of every chromosome.
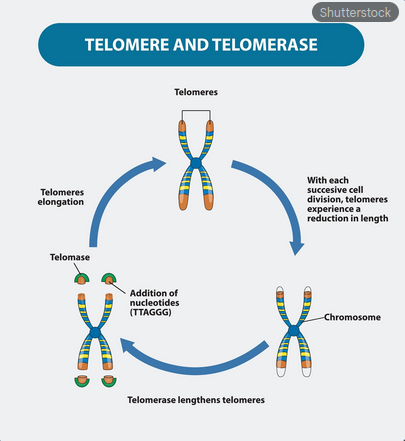
With each cellular division, these caps shorten; when they reach a critical length, the cell stops dividing and senesces, a constraint known in modern biology as the Hayflick limit.7 Tyrell, operating with what must be absolute mastery of the genome, deliberately engineered Batty’s genetic makeup to ensure this attrition occurred at a phenomenal rate, turning this natural protective mechanism into a weapon of control.
Therefore, the core of the Blade Runner conundrum is not a fault in the replication process, but a meticulously planned flaw in the genome’s maintenance system. Tyrell did not simply forget to include the necessary genetic code; he actively designed a genetic time bomb by limiting or outright suppressing the activity of Telomerase, the specialized enzyme responsible for rebuilding telomeres.8 By engineering a life-form that experienced maximal cellular turnover while simultaneously denying it the biological capacity to maintain its genetic integrity, Tyrell ensured that Batty’s body would accumulate catastrophic, unrepairable damage—the very "fatal alteration"—within exactly four years. Batty’s desperate search for a reversal is, in the simplest biological terms, a search for an injectable, systemic, and safely regulated telomerase activator, a technology that remains one of the holy grails of anti-aging science even today.
Notes
5 Scott, Ridley (Director). Blade Runner (1982). Warner Bros. Specific dialogue exchanges between Roy Batty and Dr. Eldon Tyrell.
6 Lopez-Otin, C., et al. (2013). The Hallmarks of Aging. Cell, 153(6), 1194-1215. Defining cellular senescence and genomic instability as key drivers of biological aging.
7 Blackburn, E. H. (2005). Telomeres and Telomerase: Their roles in aging and cancer. Journal of the American Medical Association, 294(20), 2634-2636. Detailed explanation of telomere function as the cellular clock.
8 Hayflick, L. (1965). The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research, 37(3), 614-636. The foundational discovery of the finite number of times a normal human cell population can divide.
The Revolution: Modern Medicine’s Answer to Tyrell
Tyrell’s assertion that any attempt to alter Batty’s core biology would be a "fatal alteration" is the scientific cornerstone of the Replicant’s tragedy. However, this claim must be re-evaluated through the lens of twenty-first-century genomic technology, which provides curative counter-evidence. The development of the CRISPR-Cas9 system—a molecular scalpel capable of editing DNA with unprecedented precision—has proven that genetic alteration is not inherently fatal; rather, it can be curative.9 Initial approvals for treatments like Casgevy, which cures Sickle Cell Disease by editing the patient’s own bone marrow cells ex vivo (outside the body), demonstrate that profound, life-altering genetic corrections are now medically routine. This breakthrough alone obliterates the categorical impossibility Tyrell cited, confirming that the flaw in Batty's genome could, in theory, be targeted and corrected.
However, Batty's plight requires a greater technological leap: systemic, in vivo editing—the ability to safely edit cells throughout the entire body, not just those sampled in a lab.10 Addressing Batty’s accelerated senescence requires repairing the telomere mechanism in his muscles, nerves, and organs. Modern science is rapidly closing this gap. Advances in delivery systems, particularly the use of Lipid Nanoparticles (LNPs), have allowed scientists to deliver genetic payloads to target organs like the liver with increasing safety and efficiency. More importantly, the evolution of the CRISPR toolkit directly addresses Tyrell’s implicit fear of mutagenic risk. Newer tools, such as Base Editors and Prime Editors, perform changes by directly converting one DNA base pair into another without cutting the double helix, dramatically minimizing the risk of unintended mutations or large-scale genetic disruptions that Tyrell feared.11
When viewed through this modern scientific panorama, the technological barrier dissolves. The problem for Roy Batty is no longer one of fundamental impossibility but one of scale and logistics. The core function required—inserting a safely regulated genetic sequence to restore telomerase activity—is achievable using current or near-future technologies that offer sub-atomic precision, far beyond the crude, early genomic tools Tyrell’s corporation might have used. This revolutionary capability transforms Batty’s tragic end from a function of inescapable science into a profound moral and corporate failure, confirming that the limit was manufactured, not ordained.12
Notes
9 Cong, L., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339(6121), 819-823. Landmark paper demonstrating the application of CRISPR-Cas9 for precise mammalian genome editing.
10 Liu, D. R. (2022). Prime Editing: Progress and Prospects. ACS Central Science, 8(8), 1109-1118. Discussing the advancement from Cas9 to Prime Editors for highly precise, non-double-strand-break edits.
11 Anzalone, A. V., et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 576(7785), 149-157. Defining the methodology and advantages of Prime Editing in reducing off-target effects.
12 T. E. F. B. G. (2020). The RNAissance of Lipid Nanoparticles. Cell, 182(2), 269-271. Review of the advancements in LNP technology for safe and effective systemic delivery of nucleic acids and editing tools.
The Telomere Paradox: Longevity’s Cost and the Cancer Trade-Off
The challenge of creating a perfect, long-lived Replicant brings us to the most complex and dangerous scientific trade-off known to biology, one that may actually offer Tyrell a shred of scientific justification: the Telomere Paradox.13 The cure for Batty's four-year limit lies in the enzyme Telomerase (hTERT), which is responsible for adding DNA sequences back to the telomeres, effectively resetting the cellular clock. In nature, this enzyme is largely silenced in most adult somatic cells, preventing uncontrolled growth. The only cells where it remains highly active are germline cells and, crucially, cancer cells. The moment a cell acquires the capacity to activate telomerase, it gains a form of biological immortality, becoming a hyper-proliferative malignancy. Therefore, Tyrell’s design flaw might not have been a lack of compassion, but a desperate and necessary precaution: granting the Replicant universal telomerase activation would be tantamount to engineering a walking, talking, guaranteed-to-die-of-cancer time bomb.14
Modern anti-aging research is currently obsessed with finding a precise way to unlock this longevity while keeping the malignant door firmly shut. We now have methods to safely activate telomerase in a temporary and non-integrating fashion, specifically using modified messenger RNA (mRNA) that codes for the hTERT subunit.15 In laboratory settings, this has successfully extended the Hayflick limit of human cells without permanently altering the host genome, allowing the telomeres to be lengthened before the mRNA degrades and the telomerase activity ceases. Furthermore, certain non-genetic interventions, such as controlled Hyperbaric Oxygen Therapy (HBOT), have demonstrated an ability to lengthen telomeres in specific populations of immune cells, suggesting that the accelerated decay of Batty’s system could be decelerated or partially reversed using existing therapies, even before radical genetic editing is deployed.
This critical safety constraint remains the one scientifically plausible defense for the Nexus-6’s hard limit. If Tyrell had failed to develop a mechanism to safely regulate telomerase activation systemically—an endeavor that remains difficult even today—then the four-year lifespan, however cruel, may have been the only window of operation before the Replicants inevitably succumbed to a cascade of aggressive, engineered cancers.16 The challenge, therefore, shifts from can we edit the genome to can we control the expression of the gene for decades across trillions of cells? While Section III demonstrated that the editing process is no longer a "fatal alteration," the regulatory follow-through remains a profound, complex, and deadly gamble that science continues to struggle with.
Notes
13 Greider, C. W., & Blackburn, E. H. (1985). Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell, 43(2), 405-413. The landmark discovery of the Telomerase enzyme.
14 Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell, 144(5), 646-674. Categorizing the sustained proliferative signaling and evasion of growth suppressors (linked to Telomerase activation) as key cancer hallmarks.
15 Jager, J. D., et al. (2020). Extending replicative lifespan of human cells by optimized mRNA delivery of telomerase reverse transcriptase. Nature Communications, 11(1), 1-13. Research detailing the temporary, non-permanent telomere lengthening using modified mRNA.
16 Aviv, A. (2012). Telomeres and Human Longevity: The Knowns and the Unknowns. BioEssays, 34(11), 946-954. Discussion on the critical balance between telomere maintenance for longevity and the inherent oncogenic risk.
Corporate Control and Ethical Tragedy
The desperate confrontation between creator and creation in Blade Runner may be immortalized as a moment of high philosophical tragedy, but when viewed through the powerful lens of contemporary molecular biology, the nature of that tragedy shifts entirely. Sections III and IV established a dual reality: the primary reason Batty died—the "fatal alteration"—was not an insurmountable scientific barrier, but a problem of precision and safety regulation that current genomic tools, such as Prime Editing and sophisticated Lipid Nanoparticle delivery systems, are now designed to overcome. While the risk of inducing systemic, aggressive cancer remains the profound counter-argument to universal telomerase activation, modern science is actively developing mRNA-based and transient gene therapies that grant the necessary temporary life extension without permanently corrupting the genome’s safety mechanisms.17
The true tragedy of the Nexus-6, therefore, lies not in scientific failure, but in human (or corporate) hubris.18 Eldon Tyrell, the architect of this engineered existence, had the technological capacity to create life that burned so brightly; yet, his refusal to grant Batty’s simple request for “more life” exposes the corporate mandate for planned obsolescence that lay at the heart of the Tyrell Corporation. The four-year limit was designed less as a biological necessity to prevent cancer and more as a crucial mechanism of control to prevent replicants from accumulating enough memory, experience, and emotional depth to become truly indistinguishable from (and uncontrollable by) humanity.
If the cure for Roy Batty's accelerated decay essentially exists today, Blade Runner transforms from a tragedy of scientific limitation into a blistering commentary on the ethics of engineered life. The film’s most enduring scientific lesson is that the most dangerous aspect of advanced biotechnology is not the inherent risk of the experiment itself, but the moral refusal of the inventor to grant grace and self-determination to the invented. The eventual development of the Nexus-7 series, a model implied to have a normal, non-accelerated lifespan, confirms that the flaw was engineered and could be corrected, forever cementing Batty's fate as a consequence of corporate tyranny, rather than scientific impossibility.19 The final question the film—and modern science—leaves us with is not whether we can build life, but whether we can be trusted with the moral responsibility of deciding how long it gets to live.
Notes
17 Zhang, Y., et al. (2021). Current status and future challenges of mRNA-based cancer immunotherapy. Acta Pharmaceutica Sinica B, 11(11), 3569-3580. Review of utilizing mRNA for therapeutic, transient protein expression in medical contexts.
18 Ridley, S. (2019). Tears in Rain. Commentary on the philosophical themes of Blade Runner and the role of the creator.
19 Dick, P. K. (1968). Do Androids Dream of Electric Sheep?. Ballantine Books. The original source material explores the varying lifespans and levels of control placed upon different synthetic human models.
ओम् तत् सत्

Member discussion: